Chapter Ten: Where The Moon and the Earth Were Joined (Two Riders Were Approaching)
 In order to understand Moore’s transformation it is necessary to fully understand precisely what Watchmen was and what it did. And to understand this it is necessary to appreciate one of its major thematic concerns, the prospect of nuclear annihilation. This aspect of the War entered the scene approximately one picosecond after the creation of the universe, when the electroweak force, unable to cohere once the universe cooled below 1015 K, split into the electromagnetic force, which would go on to underpin more or less the entirety of communication, and the weak nuclear force. This latter force was responsible for a phenomenon called beta decay, in which a neutron can transform itself into a proton by expelling an electron, along with other forms of radioactive decay. Together, along with gravity and the strong nuclear force, they create a delicate mathematical balance in which matter and life are possible. And yet within that crucial weak force is a terrifying implication.
In order to understand Moore’s transformation it is necessary to fully understand precisely what Watchmen was and what it did. And to understand this it is necessary to appreciate one of its major thematic concerns, the prospect of nuclear annihilation. This aspect of the War entered the scene approximately one picosecond after the creation of the universe, when the electroweak force, unable to cohere once the universe cooled below 1015 K, split into the electromagnetic force, which would go on to underpin more or less the entirety of communication, and the weak nuclear force. This latter force was responsible for a phenomenon called beta decay, in which a neutron can transform itself into a proton by expelling an electron, along with other forms of radioactive decay. Together, along with gravity and the strong nuclear force, they create a delicate mathematical balance in which matter and life are possible. And yet within that crucial weak force is a terrifying implication.
This implication (and indeed the weak force itself) went unnoticed for approximately fourteen billion years, until German chemists Otto Hahn and Fritz Strassman first achieved nuclear fission in 1938. Their work, of course, was simply a momentary endpoint of a larger chain of thought—the revolutions of theoretical physics that swept the scientific community in the early 20th century, upending the classical notions of the world and how it worked. Einstein, Bohr, Heisenberg, and the rest, in a series of dizzying discoveries over the course of decades, unseated the idea of a fixed and knowable universe, finding instead a universe that is fundamentally ordered by human perception, where the act of looking shapes the thing being observed—a quantum revolution that brought, in its wake, the possibility of magic. And then, with Hahn and Strassman, a very different possibility.
Hahn and Strassman conducted an experiment in which they bombarded uranium with neutrons. Observing the result, they discovered to their astonishment that the process had created atoms of barium. Reading a letter about these results, their former colleague Lisse Meitner, a Jewish chemist who had fled Germany earlier in the year, she reasoned that if Niels Bohr’s theory of the atomic nucleus, which held that it worked more or less like a drop of liquid, were correct then a very large nucleus like uranium would be unstable—indeed, unstable enough that the weak force impact of a neutron could lead the nucleus to stretch and eventually snap like a cell dividing into two. Running the calculations further, Meitner realized that the result would have less mass than the original atom, and that the lost mass must be converted into energy—indeed, a tremendous amount of energy, at least for a single atomic reaction, although on its own it was barely enough to move a speck of dust.
Word of the discovery spread quickly, and in early 1939 Niels Bohr brought word across the Atlantic as he traveled to lecture at Princeton. This set off a wave of experimentation and theorization, in which several scientists including Leó Szilárd, Enrico Fermi, and Frédéric Joliot-Curie all came to the same realization: the reaction released more than one neutron.…

 And what of Moore? Where was he as the company he forsook remade itself in what they imagined his image to be? How did the Prophet of Eternity respond to what he had wrought?
And what of Moore? Where was he as the company he forsook remade itself in what they imagined his image to be? How did the Prophet of Eternity respond to what he had wrought?
 The core of the problem was that from DC’s perspective, the lesson of Watchmen could only ever be one thing: things like this sold. And within the post-Crisis reality of the direct market, what DC specifically cared about was what fans said. The simple reality is that what the vocal fans who showed up and bought Watchmen in their specialist comic book stores liked most about the book wasn’t the moving explorations of sexuality in “A Brother To Dragons”; it was Rorschach being a moody badass in “The Abyss Gazes Also.” And so this is what DC imitated.
The core of the problem was that from DC’s perspective, the lesson of Watchmen could only ever be one thing: things like this sold. And within the post-Crisis reality of the direct market, what DC specifically cared about was what fans said. The simple reality is that what the vocal fans who showed up and bought Watchmen in their specialist comic book stores liked most about the book wasn’t the moving explorations of sexuality in “A Brother To Dragons”; it was Rorschach being a moody badass in “The Abyss Gazes Also.” And so this is what DC imitated. Consider January of 1987, the month that Moore finally came to the decision that he would not accept any new work from DC. In addition to Watchmen #8, in which Rorschach breaks out of prison with the help of Dan and Laurie after violently murdering his way through a number of inmates, high profile comics being released included the final issue of Legends, DC’s first attempt to duplicate the success of Crisis on Infinite Earths, two issues of John Byrne’s Superman run, and an issue of George Pérez’s Wonder Woman. In more grim and gritty terms, DC released an issue of Vigilante, with its right-wing fantasy of extrajudicial executions, and The Question, which saw Denny O’Neil putting his own spin on Rorschach’s source material, and the third installment of Frank Miller’s post-Crisis Batman: Year One relaunch. But even within the Batman line, that same month saw the release of Detective Comics #573, a goofball issue featuring the Mad Hatter, albeit one that ends with Robin being shot and gravely wounded. But DC still had at least one eye firmly situated on the past. Other titles included Infinity Inc., co-written by Roy Thomas, a twenty-year veteran of the industry who had succeeded Stan Lee as editor in chief at Marvel Comics.…
Consider January of 1987, the month that Moore finally came to the decision that he would not accept any new work from DC. In addition to Watchmen #8, in which Rorschach breaks out of prison with the help of Dan and Laurie after violently murdering his way through a number of inmates, high profile comics being released included the final issue of Legends, DC’s first attempt to duplicate the success of Crisis on Infinite Earths, two issues of John Byrne’s Superman run, and an issue of George Pérez’s Wonder Woman. In more grim and gritty terms, DC released an issue of Vigilante, with its right-wing fantasy of extrajudicial executions, and The Question, which saw Denny O’Neil putting his own spin on Rorschach’s source material, and the third installment of Frank Miller’s post-Crisis Batman: Year One relaunch. But even within the Batman line, that same month saw the release of Detective Comics #573, a goofball issue featuring the Mad Hatter, albeit one that ends with Robin being shot and gravely wounded. But DC still had at least one eye firmly situated on the past. Other titles included Infinity Inc., co-written by Roy Thomas, a twenty-year veteran of the industry who had succeeded Stan Lee as editor in chief at Marvel Comics.…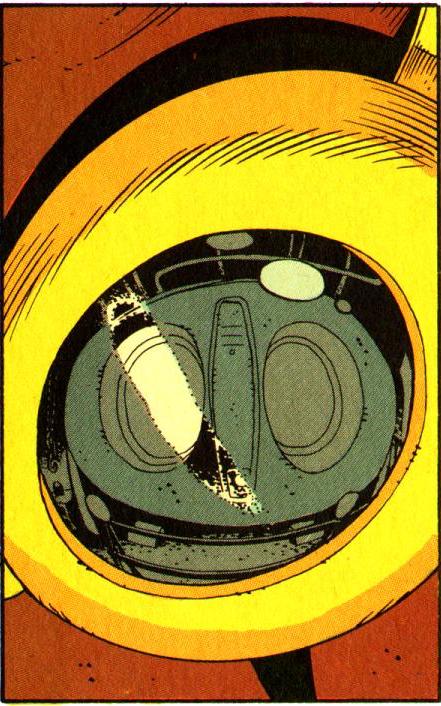 There is, however, another important sense in which Rorschach represents a myopia within Watchmen and, more broadly, Moore’s larger artistic vision. As mentioned, a crucial part of Rorschach’s psychology is his tortured relationship with sexuality. Sex is a major theme of both Watchmen and Moore’s career, and one that he has much of value to say about, but there is something unseemly about the directness with which Rorschach’s disgust with sex is pathologized, not least because it’s a character trait inherited from his underlying relationship with the apparently asexual Steve Ditko. More broadly, there is something oversimplified and unsatisfying in Moore’s approach to sexuality—a flaw intimately connected to his persistent inadequacy on the subject of sexual assault. This would be a relatively minor issue were it not for the awkward fact that the relationship between superheroes and sexuality is one of the comic’s major themes.
There is, however, another important sense in which Rorschach represents a myopia within Watchmen and, more broadly, Moore’s larger artistic vision. As mentioned, a crucial part of Rorschach’s psychology is his tortured relationship with sexuality. Sex is a major theme of both Watchmen and Moore’s career, and one that he has much of value to say about, but there is something unseemly about the directness with which Rorschach’s disgust with sex is pathologized, not least because it’s a character trait inherited from his underlying relationship with the apparently asexual Steve Ditko. More broadly, there is something oversimplified and unsatisfying in Moore’s approach to sexuality—a flaw intimately connected to his persistent inadequacy on the subject of sexual assault. This would be a relatively minor issue were it not for the awkward fact that the relationship between superheroes and sexuality is one of the comic’s major themes. The theme of sex within Watchmen ignites in the seventh issue, “A Brother to Dragons,” which forms, along with “The Abyss Gazes Also,” a symmetrical axis at the center of the series. Watchmen can be divided into two types of issues: ensemble pieces that feature a large cross-section of the cast, and character-focused issues that provide backgrounds and meditations on individual heroes. The first half of the book alternates between these two types, with “At Midnight, All the Agents,” “The Judge of All the Earth,” and “Fearful Symmetry” jumping among multiple points of view while “Absent Friends,” “Watchmaker,” and “The Abyss Gazes Also” focus on the Comedian, Dr. Manhattan, and Rorschach respectively. The second half also alternates back and forth, but here it is the odd-numbered issues that are character-focused, looking at Ozymandias, Silk Spectre, and, in “A Brother To Dragons,” Night Owl.
The theme of sex within Watchmen ignites in the seventh issue, “A Brother to Dragons,” which forms, along with “The Abyss Gazes Also,” a symmetrical axis at the center of the series. Watchmen can be divided into two types of issues: ensemble pieces that feature a large cross-section of the cast, and character-focused issues that provide backgrounds and meditations on individual heroes. The first half of the book alternates between these two types, with “At Midnight, All the Agents,” “The Judge of All the Earth,” and “Fearful Symmetry” jumping among multiple points of view while “Absent Friends,” “Watchmaker,” and “The Abyss Gazes Also” focus on the Comedian, Dr. Manhattan, and Rorschach respectively. The second half also alternates back and forth, but here it is the odd-numbered issues that are character-focused, looking at Ozymandias, Silk Spectre, and, in “A Brother To Dragons,” Night Owl. The line, of course, belongs to Rorschach, the glamorous poison at the heart of Watchmen’s appeal. Moore is self-effacing about the character these days, joking that his popularity is down to the fact that Moore “had forgotten that actually to a lot of comic fans that smelling, not having a girlfriend – these are actually kind of heroic.” But unlike a lot of Moore’s self-deprecation, there’s an edge to this quip. He’s emphasizing his failure to anticipate the reaction to Rorschach, but only as a means to insult Rorschach’s fans even more spectacularly. There are obviously a lot of things about Watchmen that have gone sour for Moore, but at times it seems that there is nothing he resents quite as deeply as the reception of Rorschach.
The line, of course, belongs to Rorschach, the glamorous poison at the heart of Watchmen’s appeal. Moore is self-effacing about the character these days, joking that his popularity is down to the fact that Moore “had forgotten that actually to a lot of comic fans that smelling, not having a girlfriend – these are actually kind of heroic.” But unlike a lot of Moore’s self-deprecation, there’s an edge to this quip. He’s emphasizing his failure to anticipate the reaction to Rorschach, but only as a means to insult Rorschach’s fans even more spectacularly. There are obviously a lot of things about Watchmen that have gone sour for Moore, but at times it seems that there is nothing he resents quite as deeply as the reception of Rorschach. The fundamental problem at the heart of this is simply that Rorschach is an unsettlingly fascinating character. Even Moore, for all the reservations he would come to have about him, freely admits that “Rorschach was one of the characters I enjoyed writing most.” And no wonder – think of a classic line in Watchmen and the odds are overwhelming that you’re thinking of a Rorschach line, probably either a bit of his opening monologue or “none of you understand. I’m not locked up in here with you. You’re locked up in here with me.” Whether or not one relates to the character, he is undeniably compelling, reliably giving the book a jolt of energy whenever he shows up.
The fundamental problem at the heart of this is simply that Rorschach is an unsettlingly fascinating character. Even Moore, for all the reservations he would come to have about him, freely admits that “Rorschach was one of the characters I enjoyed writing most.” And no wonder – think of a classic line in Watchmen and the odds are overwhelming that you’re thinking of a Rorschach line, probably either a bit of his opening monologue or “none of you understand. I’m not locked up in here with you. You’re locked up in here with me.” Whether or not one relates to the character, he is undeniably compelling, reliably giving the book a jolt of energy whenever he shows up._p01_(comichost-dcp).jpg) Moore’s disorientation and confusion in the wake of Watchmen is wholly understandable. Even reading Watchmen is, at times, enough to generate a sense of dazed exhaustion. And this is very much the point – an effect consciously generated by Moore’s use of the dense uniformity of the nine-panel grid. As Kieron Gillen puts it in Kieron Gillen Talks Watchmen, “if we’re talking about the many icons of Watchmen, [the nine-panel grid] is the invisible one. It underlies everything. We’re to watch these little boxes – hundreds of them – and make sense by combining them all into a larger piece of meaning. Watch,” he says, and snaps his fingers to cue his projectionist to advance his PowerPoint to a shot of Ozymandias watching his wall of television screens. Gillen talks about the comic as a “clockwork machine” in which “everything is predetermined. The forces that are put into motion mean this… the clock will carry on ticking, and if you read Watchmen enough you’ll know what the next tick is.” Gillen, here, is talking about the comic’s famously ambiguous ending, making a strong case that in fact there is only one possible “next step” for the book to take, and that the inevitable momentum of that step hangs impermeably over the entire work, which is in turn what Morrison speaks of when he talks about how the god of Watchmen is always shoving his cock in the reader’s face.
Moore’s disorientation and confusion in the wake of Watchmen is wholly understandable. Even reading Watchmen is, at times, enough to generate a sense of dazed exhaustion. And this is very much the point – an effect consciously generated by Moore’s use of the dense uniformity of the nine-panel grid. As Kieron Gillen puts it in Kieron Gillen Talks Watchmen, “if we’re talking about the many icons of Watchmen, [the nine-panel grid] is the invisible one. It underlies everything. We’re to watch these little boxes – hundreds of them – and make sense by combining them all into a larger piece of meaning. Watch,” he says, and snaps his fingers to cue his projectionist to advance his PowerPoint to a shot of Ozymandias watching his wall of television screens. Gillen talks about the comic as a “clockwork machine” in which “everything is predetermined. The forces that are put into motion mean this… the clock will carry on ticking, and if you read Watchmen enough you’ll know what the next tick is.” Gillen, here, is talking about the comic’s famously ambiguous ending, making a strong case that in fact there is only one possible “next step” for the book to take, and that the inevitable momentum of that step hangs impermeably over the entire work, which is in turn what Morrison speaks of when he talks about how the god of Watchmen is always shoving his cock in the reader’s face. It is January, 1988. Alan Moore is thirty-three, and writing the introduction to the collected edition of Watchmen. His relationship with the publisher is in tatters, a fact he alludes to only vaguely when he notes that it is “the very last work that I expect to be doing upon Watchmen for the foreseeable future.” He looks back to 1984, when the idea originated, and the giddy enthusiasm of it all. And yet there is something he cannot quite locate in this. He notes that at the start “we wanted to do a novel and unusual super-hero comic that allowed us the opportunity to try out a few new storytelling ideas along the way. What we ended up with was substantially more than that.” But he cannot pin down the transition. Instead he describes a growing realization of the story’s complexity and scale, but without a sense that the realization had an end point. Instead, “there was the mounting suspicion (at least on my part) that we might have bitten off more than we could chew, that we might not be able to resolve all these momentous strands of narrative and meaning that seemed to be springing up wherever we looked, that we might be left at the end of the day with a big, messy, steaming bowl of semiotic spaghetti.” But even now, in 1988, Moore is lost in the process, saying that it is only now, in writing the introduction, that he realizes “how dazed a state I’d spent that year in, as if I’d been slam dancing with a bunch of rhinos and the concussion was only just starting to clear up,” a claim that oddly undercuts itself; the daze clearly extending beyond the year, simultaneously located “now, twelve months after” he finished the final script and in the past, as indicated by the past tense in the concussion metaphor.
It is January, 1988. Alan Moore is thirty-three, and writing the introduction to the collected edition of Watchmen. His relationship with the publisher is in tatters, a fact he alludes to only vaguely when he notes that it is “the very last work that I expect to be doing upon Watchmen for the foreseeable future.” He looks back to 1984, when the idea originated, and the giddy enthusiasm of it all. And yet there is something he cannot quite locate in this. He notes that at the start “we wanted to do a novel and unusual super-hero comic that allowed us the opportunity to try out a few new storytelling ideas along the way. What we ended up with was substantially more than that.” But he cannot pin down the transition. Instead he describes a growing realization of the story’s complexity and scale, but without a sense that the realization had an end point. Instead, “there was the mounting suspicion (at least on my part) that we might have bitten off more than we could chew, that we might not be able to resolve all these momentous strands of narrative and meaning that seemed to be springing up wherever we looked, that we might be left at the end of the day with a big, messy, steaming bowl of semiotic spaghetti.” But even now, in 1988, Moore is lost in the process, saying that it is only now, in writing the introduction, that he realizes “how dazed a state I’d spent that year in, as if I’d been slam dancing with a bunch of rhinos and the concussion was only just starting to clear up,” a claim that oddly undercuts itself; the daze clearly extending beyond the year, simultaneously located “now, twelve months after” he finished the final script and in the past, as indicated by the past tense in the concussion metaphor. A similar temporal ambivalence exists in the previously discussed description of how his “nostalgia for the [superhero] genre cracked and shattered somewhere along the way and all the sweet old musk inside just leaked out and evaporated,” a statement in which Moore is clearly lying, albeit as much to himself as the reader, the description of the “sweet old musk” evoking precisely what he says he has lost, so that the sentence offers the very experience it exists to reject. Moore casts superheroes away, but in doing so only draws them back in, a tension implicit in the very idea of nostalgia, which is of course an intense feeling for a thing that one is not actually experiencing at the time. He is nostalgic for his nostalgia, relishing in the scent of its memory even as he pushes the genre away, grimly certain in the necessity of all his burnt bridges. The “disappointment when I learned that we couldn’t use the Charlton characters after all” mixes in his prose with the bitter taste of his falling out with DC.
A similar temporal ambivalence exists in the previously discussed description of how his “nostalgia for the [superhero] genre cracked and shattered somewhere along the way and all the sweet old musk inside just leaked out and evaporated,” a statement in which Moore is clearly lying, albeit as much to himself as the reader, the description of the “sweet old musk” evoking precisely what he says he has lost, so that the sentence offers the very experience it exists to reject. Moore casts superheroes away, but in doing so only draws them back in, a tension implicit in the very idea of nostalgia, which is of course an intense feeling for a thing that one is not actually experiencing at the time. He is nostalgic for his nostalgia, relishing in the scent of its memory even as he pushes the genre away, grimly certain in the necessity of all his burnt bridges. The “disappointment when I learned that we couldn’t use the Charlton characters after all” mixes in his prose with the bitter taste of his falling out with DC. But more important than the way these things slide away from him is what he is looking for in amidst the rubble: “any sort of perspective upon what it was we actually did.”…
But more important than the way these things slide away from him is what he is looking for in amidst the rubble: “any sort of perspective upon what it was we actually did.”… Part of the difficulty in tracking the fallout of Moore’s split with DC is simply that it’s so extensive. Once the rupture began it spread quickly, fueled in no small part by Alan Moore’s tendency to, as he’s put it, burn his bridges when reacting against something so as to make sure he’s never tempted to go second guess himself. But in this case the fractal repetition of Moore’s grievances with DC have served to make the initial issues harder to see, to the point where the standard wisdom is that Moore’s break with DC came over a dispute in the handling of the Watchmen trade paperback when, in reality, he had made his decision not to accept any new work from DC in January of 1987, eight months before the trade paperback even came out, and five months before Moore was actually done working on it. This decision came during a wider dispute about DC’s proposed creation of a ratings system for their comics. And even that point is not a single discrete cause that can be separated out from all of the others and identified as the original, true rift, but at best the third issue to arise between Moore and DC in quick succession.
Part of the difficulty in tracking the fallout of Moore’s split with DC is simply that it’s so extensive. Once the rupture began it spread quickly, fueled in no small part by Alan Moore’s tendency to, as he’s put it, burn his bridges when reacting against something so as to make sure he’s never tempted to go second guess himself. But in this case the fractal repetition of Moore’s grievances with DC have served to make the initial issues harder to see, to the point where the standard wisdom is that Moore’s break with DC came over a dispute in the handling of the Watchmen trade paperback when, in reality, he had made his decision not to accept any new work from DC in January of 1987, eight months before the trade paperback even came out, and five months before Moore was actually done working on it. This decision came during a wider dispute about DC’s proposed creation of a ratings system for their comics. And even that point is not a single discrete cause that can be separated out from all of the others and identified as the original, true rift, but at best the third issue to arise between Moore and DC in quick succession. Certainly Moore would have had little reason to see DC as essential to his career. He had, after all, succeeded with essentially every company he’d worked with.…
Certainly Moore would have had little reason to see DC as essential to his career. He had, after all, succeeded with essentially every company he’d worked with.… This is, perhaps, why so much ink has been spilled within the War on attempts to argue that this gap, in effect, does not exist – that Watchmen can be understood purely, or at least primarily, in terms of its influences, thus allowing those living in its wake to exist as though they are free from its vast and monolithic splendor. It is, after all, the easier option; it does not require staring too long at the cavernous depths within. It gives the comforting illusion that Watchmen is, at its heart, an easily solved mystery – a question with a definite answer. Nothing could be further from the truth, but for those who would otherwise find themselves caught in its blast, reduced to mere shadows cast by its incinerating radiance the idea that the book is simply some inevitable consequence of what came before is a useful delusion.
This is, perhaps, why so much ink has been spilled within the War on attempts to argue that this gap, in effect, does not exist – that Watchmen can be understood purely, or at least primarily, in terms of its influences, thus allowing those living in its wake to exist as though they are free from its vast and monolithic splendor. It is, after all, the easier option; it does not require staring too long at the cavernous depths within. It gives the comforting illusion that Watchmen is, at its heart, an easily solved mystery – a question with a definite answer. Nothing could be further from the truth, but for those who would otherwise find themselves caught in its blast, reduced to mere shadows cast by its incinerating radiance the idea that the book is simply some inevitable consequence of what came before is a useful delusion.
